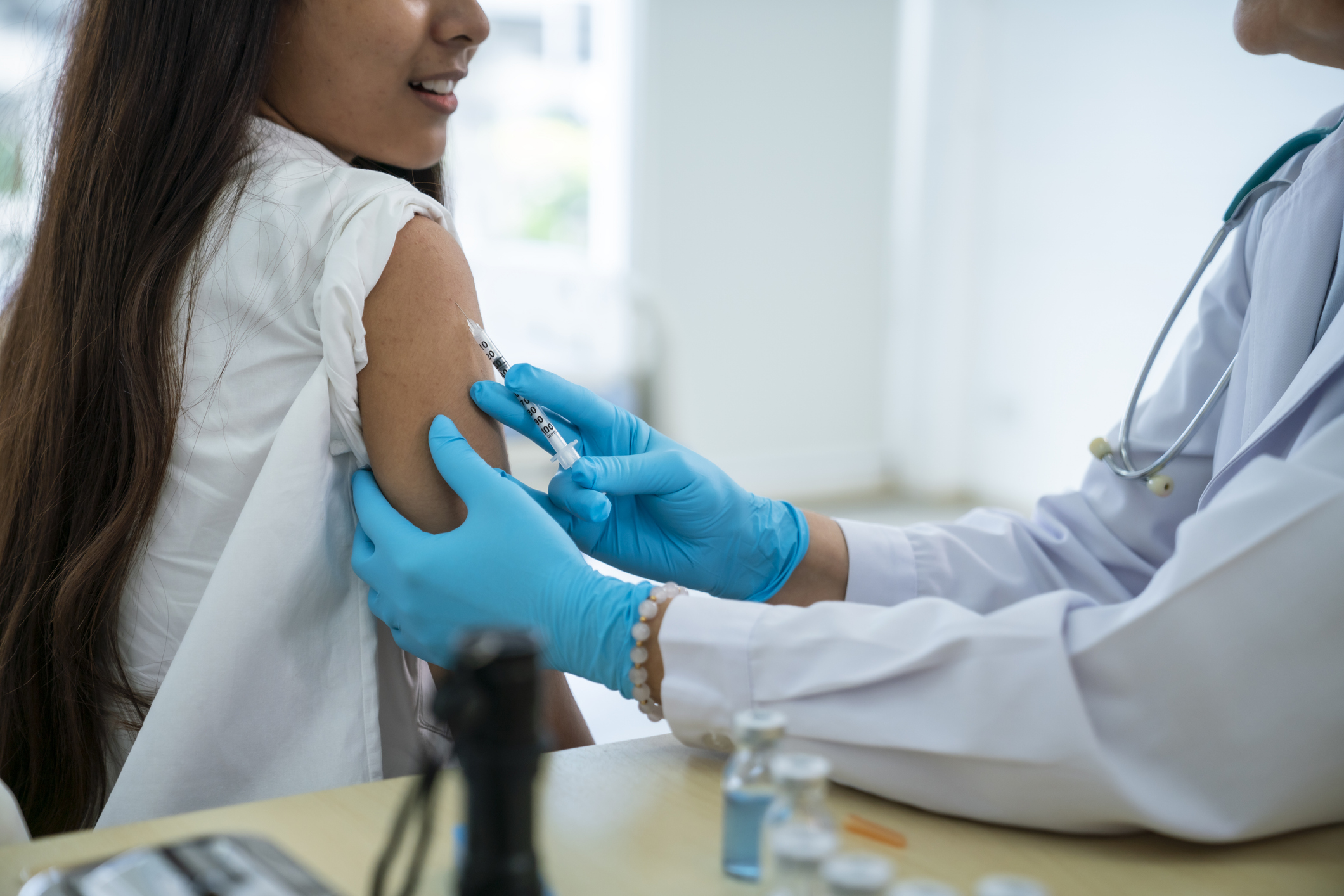
The Union Ministry of Health & Family Welfare has approved the introduction of a novel treatment regimen for multidrug-resistant tuberculosis (MDR-TB) under its National TB Elimination Program (NTEP).
The new BPaLM regimen, a four-drug combination of Bedaquiline, Pretomanid, Linezolid, and Moxifloxacin, promises to revolutionize MDR-TB treatment in India. This shorter, six-month treatment option is set to replace the traditional 20-month procedure, offering higher success rates with fewer side effects.
This treatment will benefit approximately 75,000 drug-resistant TB patients in India. It not only significantly reduces treatment time, but also promises substantial cost savings.
The decision follows a rigorous review process involving in-country subject experts and a Health Technology Assessment to ensure the regimen’s safety and cost-effectiveness. The Central TB Division is now preparing a nationwide roll-out plan, which includes comprehensive training for health professionals.
This initiative aligns with Prime Minister Narendra Modi’s vision to eliminate TB in India by 2025, five years ahead of the global Sustainable Development Goals target. It builds upon recent efforts like the Pradhan Mantri TB Mukt Bharat Abhiyaan and the Ni-kshay Mitra initiative, launched by President Droupadi Murmu in September 2022.
India has the world’s largest TB laboratory network with 7,767 rapid molecular testing facilities and 87 culture and drug susceptibility testing laboratories spread across the length and breadth of the country. This widespread laboratory network will support in timely detection of MDR-TB and quick initiation of TB treatment.