The Indian Council of Medical Research (ICMR) and Panacea Biotec have initiated the first-ever Phase 3 clinical trial for a dengue vaccine in the country. The trial, which commenced on Wednesday at PGIMS Rohtak, will evaluate the efficacy of DengiAll, India’s indigenous tetravalent dengue vaccine developed by Panacea Biotec.
Union Health Minister J P Nadda hailed the development as a “critical advancement” in India’s fight against dengue. “This collaboration between ICMR and Panacea Biotec not only aims to protect our citizens but also reinforces our vision of Atmanirbhar Bharat in healthcare,” Nadda said.
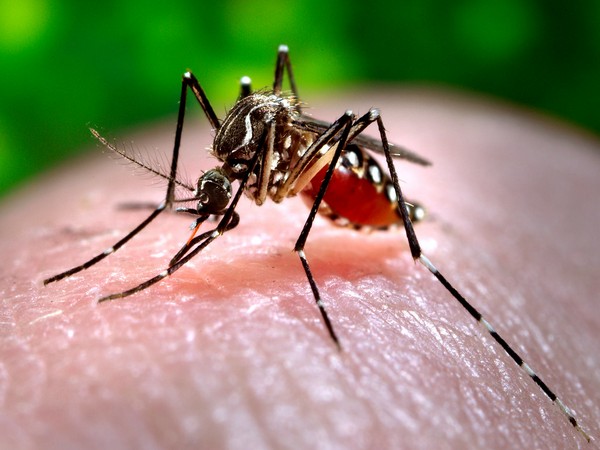
The trial comes at a crucial time, as India currently lacks any antiviral treatment or licensed vaccine for dengue. The disease, caused by four distinct serotypes, poses a complex challenge for vaccine development. India is among the top 30 countries with the highest dengue incidence, with the WHO reporting a steady global increase over the past two decades.
DengiAll is based on a tetravalent dengue vaccine strain (TV003/TV005) originally developed by the U.S. National Institutes of Health. Panacea Biotec, one of three Indian companies to receive the strain, has conducted extensive research to develop a full-fledged vaccine formulation, completing Phase 1 and 2 trials in 2018-19 with promising results.
In collaboration with ICMR, Panacea Biotec will conduct the Phase 3 clinical trial across 19 sites in 18 States and Union Territories of India, involving more than 10,335 healthy adult participants. The trial, primarily funded by ICMR with partial support from Panacea Biotec, is set to follow up with participants for two years. This initiative represents a significant step towards developing an indigenous vaccine for one of India’s most pressing public health challenges and exemplifies the nation’s commitment to Atmanirbhar Bharat.
ICMR will primarily fund the trial, with partial support from Panacea Biotec. Participants will be followed up for two years.
Dr. Rajesh Bhushan, Secretary of the Department of Health and Family Welfare, emphasized the trial’s importance: “This initiative represents a significant step towards addressing one of India’s most pressing public health challenges. It showcases our commitment to indigenous vaccine development and self-reliance in healthcare.”
Dengue is a major public health concern in India, ranking among the top 30 countries with the highest incidence of the disease. The global incidence of dengue has been steadily increasing over the past two decades, with more than 129 countries reporting dengue viral disease by the end of 2023, according to the World Health Organization (WHO).
In India, approximately 75-80 per cent of infections are asymptomatic, yet these individuals can still transmit the infection through the bite of Aedes mosquitoes. Among the 20-25 per cent of cases where symptoms are clinically apparent, children are at a significantly higher risk of hospitalization and mortality.
In adults, the disease can escalate into severe conditions like dengue hemorrhagic fever and dengue shock syndrome. The dengue virus has four serotypes, 1-4, with low cross-protection against each other, meaning individuals can experience repeated infections.
(Inputs from ANI)