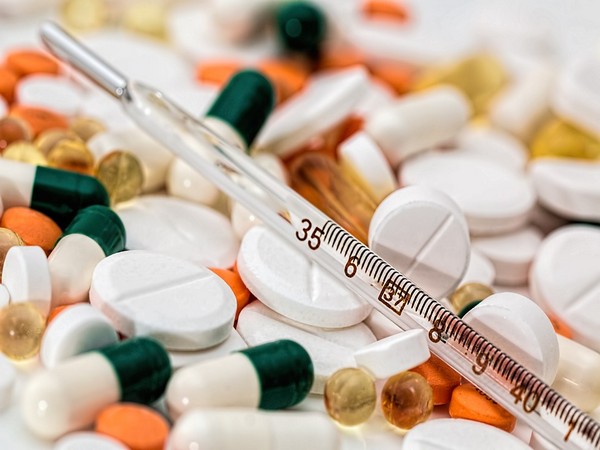
The central government has waived the requirement for local clinical trials for several drugs in India. According to official sources, medicines that have already been approved by regulatory authorities in countries such as the US, UK, and EU, and are not readily available in India, are now exempt from the need for local trials.
“Currently, several medicines already approved by other regulatory authorities like the US, UK, and EU are not immediately available for Indian patients due to certain regulatory requirements under the Drugs and Cosmetics Act and the rules made thereunder. These requirements include conducting a local clinical trial and generating safety and efficacy data locally before marketing authorization in India,” said official sources.
The government has taken this decision to prevent delays in launching these drugs.
Based on patient needs, it has now been decided that certain countries like the USA, UK, Japan, Australia, Canada, and those in the EU can supply drugs to India without requiring local trials under Rule 101, subject to certain conditions.
This exemption applies to drugs such as orphan drugs for rare diseases, gene and cellular therapy products, new drugs used in pandemic situations, new drugs for special defense purposes, and new drugs that offer significant therapeutic advances over the current standard of care.
“The latest medicines to treat diseases like cancer, rare diseases such as SMA and DMA, and autoimmune conditions will now become available more quickly in India,” said official sources.
Furthermore, official sources indicated that this decision will help reduce the costs of public procurement by the Indian government and state governments under various schemes like CGHS and Ayushman Bharat.
“The costs incurred by pharmaceutical firms in conducting local clinical trials will be reduced, and these savings can be passed on to patients,” sources added.
However, final phase four clinical trials for these drugs will still be mandatory.
Source: ANI