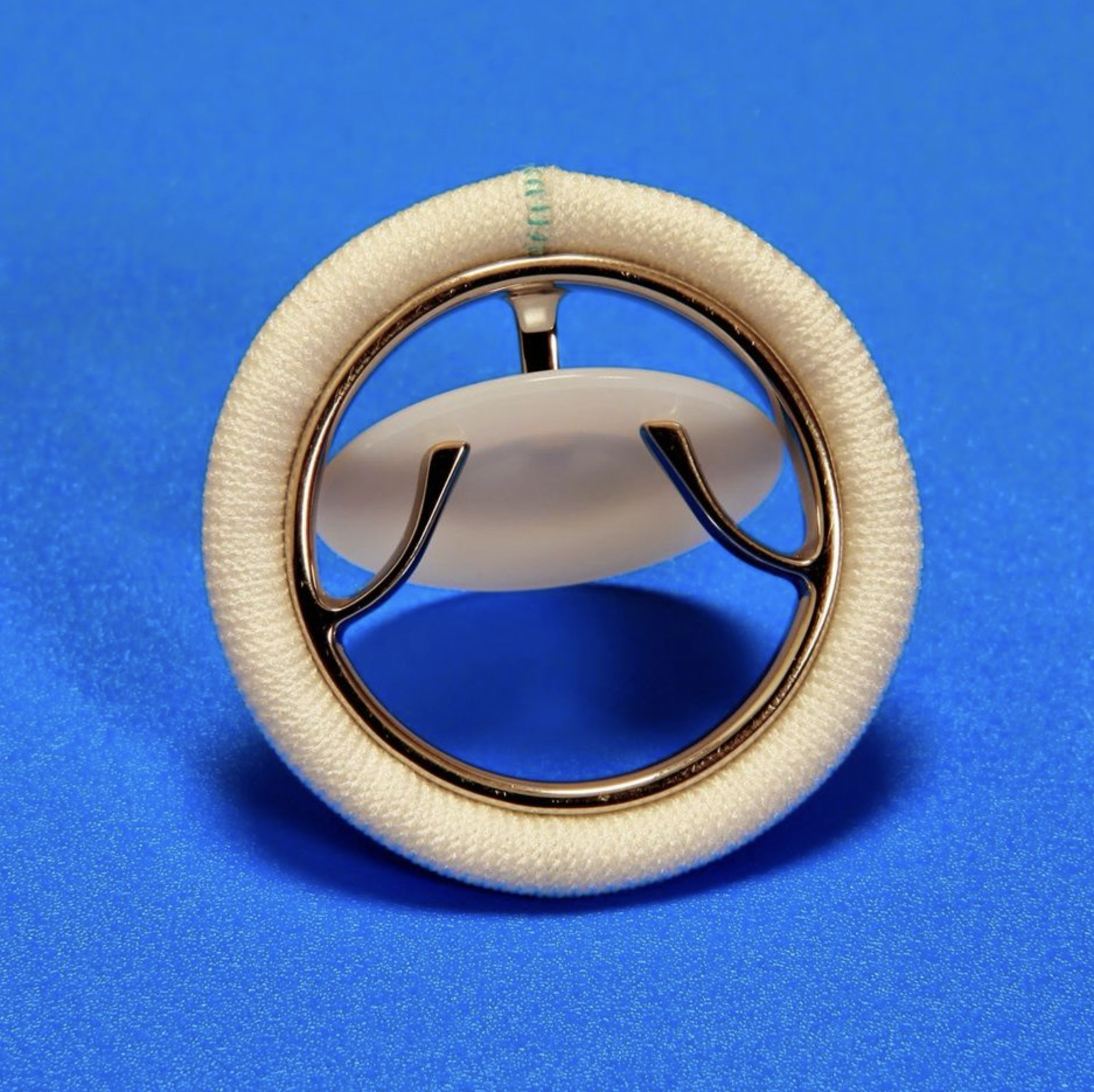
The low-cost Sree Chitra valve is an example of how a successful implementation of the innovation ecosystem is helping India tackle Rheumatic heart disease and contributing to the country’s march towards Swasth Bharat.
While the first Chitra Heart Valve was successfully implanted in a patient in 1990, subsequent efforts to improve upon and scale up the product have led to the clinical use of close to 2,00,000 devices with success rates comparable to any other heart valve in the market today.
Rheumatic heart disease, which leads to the damage of heart valves needing prosthetic replacement, is a challenge in India. In the 1980s, based on the estimate of the Indian Council for Medical Research, 6 out of every 1000 children had rheumatic fever and the young population at risk for valvular disease was estimated at 12 lakhs. The valves which are the solution to the disease, needed to be imported at a very high cost and were not affordable to all.
Responding to India’s requirement for low-cost indigenous artificial heart valves Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), an autonomous institute of the Department of Science and Technology (DST) put together a multidisciplinary team to take up the development challenge.
The valve was developed, and the first implant was carried out in 1990. While the patient has completed about three decades of healthy life since then, the valve has been improved over time to ensure better functioning and durability.
The first-generation Chitra heart valve model had an ultrahigh molecular weight polyethylene (UHMW-PE) disc; a Haynes-25 alloy cage and a sewing ring fabricated from warp knitted polyethylene terephthalate (PET). The basic features of the design included free floating disc that could rotate on its central axis to minimize the problem of thrombosis formation around the hinge area as well as distribute the wear generated during its functioning over a larger surface; a planoconvex disc with an inlet side flat for enhanced hemodynamics; lower levels of impact forced during the closure of the valve compared to other mechanical valves, leading to lower sound levels during operation as well as reduced disturbance in the tissue metal interface; reduced cavitation damage potential achieved by the choice of soft UHMWPE disc material.
Based on the feedback from the clinical trials of the earlier model, and post-market surveillance, few improvements were identified and implemented in the second-generation device. These include enhanced MRI compatibility by incorporating Titanium alloy instead of Haynes 25 used in the earlier model, improved thrombo-resistance, and reduced bare metal exposure to the bloodstream by coating Titanium nitride (TiN) on the frame surface, Larger Effective Orifice Area (EOA) and hemodynamic performance achieved through design modifications.
These are expected to improve the quality of life of the patient in terms of enhanced performance and reduced complications.
After obtaining necessary regulatory approvals from the Central Drug Standards Control (CDSCO), the pilot clinical evaluation of this model TC2 was initiated in SCTIMST in the last two years and forty valves were implanted in patients. The results are observed to be very promising and no specific complications were reported. Based on this positive outcome, a pivotal clinical trial has been planned and is expected to start by the end of 2024.
With prophylaxis at the community level being strengthened, it is expected that within a couple of decades, the primary cause of heart valve replacement will shift from rheumatic heart disease to degenerative diseases. And, accordingly, the number of elderly people with the requirement for valve surgeries is likely to increase. This would increase the need for tissue-based heart valves in India. The Institute initiated a tissue valve development program under the Technical Research Centre (TRC) for Biomedical Devices program in 2019.
The prototyping and preliminary proof of concept studies have been completed. The institute has initiated the identification of a suitable industrial partner for taking this product to the clinic which is expected by 2026.
The journey of development and commercialization of this frugal innovation is an example of how an ecosystem leader co-created the innovation ecosystem that led to the development of a low-cost heart valve by engaging in three types of configuration boundary work – establishing ecosystem configuration, modelling ecosystem configuration, and expanding ecosystem configuration, according to a paper published in October last year in the journal of Product Innovation Management. It is also an exemplary step in India’s journey of inclusive health care through frugal innovations.
The core team from the Institute is currently being led by Shri C.V. Muraleedharan from the Department of Medical Devices Engineering, Dr. P.R. Umashankar from the Department of Applied Biology, and Dr. Vivek V. Pillai from the Department of Cardiovascular Surgery.
Tapas Bhattacharya's Report